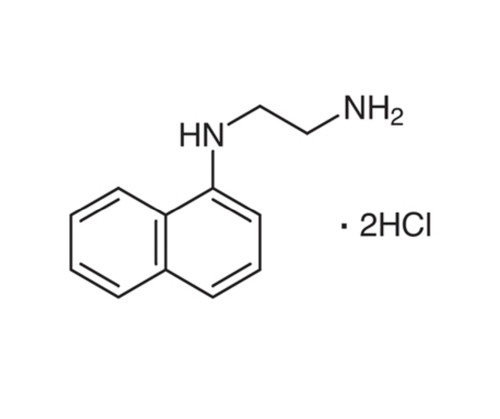
N (1-Naphthyl)Ethylenediamine Dihydrochloride
500 INR/Kilograms
Product Details:
- Grade Analytical Grade
- Smell Odorless
- Poisonous Non-poisonous
- Physical Form Solid
- Melting Point 220-225C (dec)
- Taste Odorless
- Molecular Formula C12H16Cl2N2
- Click to View more
X
N (1-Naphthyl)Ethylenediamine Dihydrochloride Price And Quantity
- 500 INR/Kilograms
- 100 Kilograms
N (1-Naphthyl)Ethylenediamine Dihydrochloride Product Specifications
- 215-972-6
- 1.25 Gram per cubic centimeter(g/cm3)
- 259.18 g/mol
- Crystalline
- Freely soluble in water
- White to Off-white Powder
- 1465-25-4
- 29223990
- Non-poisonous
- Odorless
- Analytical Grade
- 98%
- Solid
- Odorless
- C12H16Cl2N2
- 220-225C (dec)
- 5.0 - 6.0 (1% solution)
- C10H7NHCH2CH2NH22HCl
- N-(1-Naphthyl)ethane-1,2-diamine dihydrochloride
- Not determined
N (1-Naphthyl)Ethylenediamine Dihydrochloride Trade Information
- 100000 Kilograms Per Day
- 1 Days
Product Description
| Property | Value |
| Specification | N-(1-Naphthyl)Ethylenediamine Dihydrochloride (NEDA) |
| Other names | NEDA chemical, N1-NAPTHYL ETHYLENE DIAMINE DIHYDROCHLORIDE |
| CAS | 1465-25-4 |
| Molecular Formula | C12H14N2.2HCl |
| Molecular Weight | 259.18 |
| Appearance | White to off white powder or crystal |
| Loss on drying | Max. 5% |
| Assay (On dry basis) | Min. 98% |
| M.P. | 190-197C |
| Sulphated Ash | Max. 0.1% |
Usage in Analytical Chemistry
N (1-Naphthyl)Ethylenediamine Dihydrochloride is valued for its sensitivity in colorimetric assays to detect nitrite and nitrate concentrations. The chemical forms colored complexes upon reaction, allowing for precise qualitative and quantitative analyses. Its reliability and ease of solubility in water enhance convenience in both laboratory and industrial testing environments.
Proper Storage and Stability
To maintain stability, this reagent must be stored in a tightly closed container, away from light and moisture, at room temperature. Common packaging includes sealed amber bottles or HDPE containers. Under these storage conditions, the product remains stable for up to two years, ensuring consistent results throughout its shelf life.
Safety and Handling Precautions
Although NED Dihydrochloride is non-poisonous and odorless, it is essential to utilize appropriate personal protective equipment (PPE) and avoid contact with eyes, skin, or clothing. Good laboratory practices, including storing away from incompatible substances and minimizing exposure to the environment, are recommended for safe usage.
FAQ's of N (1-Naphthyl)Ethylenediamine Dihydrochloride:
Q: How should N (1-Naphthyl)Ethylenediamine Dihydrochloride be stored to ensure longevity?
A: Store the compound in a tightly closed container protected from light and moisture, at room temperature. Proper storage in sealed amber bottles or HDPE containers maintains its stability and extends its shelf life to up to two years from the date of manufacture.Q: What is the main usage of this reagent in analytical chemistry?
A: The primary application is as a colorimetric reagent for detecting nitrites and nitrates in various samples. It forms colored complexes that facilitate both qualitative and quantitative analyses in environmental, pharmaceutical, and food laboratories.Q: When does the product become unsuitable for use?
A: NED Dihydrochloride should not be used beyond its two-year shelf life or if signs of exposure to moisture or light-such as discoloration or clumping-are observed. Adhering to recommended storage conditions ensures consistent performance.Q: Where is this product commonly utilized?
A: This reagent is widely employed in environmental monitoring labs, water treatment facilities, and chemical analysis laboratories across research and industrial sectors, particularly in India.Q: What process is involved in using this compound for nitrite detection?
A: Typically, the reagent is dissolved in water and added to the sample, where it reacts with nitrites to produce a colored compound. This color development is measured colorimetrically to determine nitrite concentration.Q: What are the safety precautions when handling NED Dihydrochloride?
A: Personal protective equipment, such as gloves and safety goggles, should be worn. Avoid direct contact with eyes, skin, and clothing, and handle the reagent in a well-ventilated area.Q: What are the benefits of choosing this reagent for laboratory analysis?
A: With high purity, excellent water solubility, and stability when stored correctly, this reagent offers consistent, accurate results for colorimetric assays and is simple to handle and implement in various analytical protocols.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Fine Chemicals' category
 |
SUVIDHINATH LABORATORIES
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |